Classification

In the Standard Model of particle physics, electrons belong to the group of subatomic particles called leptons, which are believed to be fundamental or elementary particles. Electrons have the lowest mass of any charged lepton (or electrically charged particle of any type) and belong to the first-generation of fundamental particles.[64] The second and third generation contain charged leptons, the muon and the tauon, which are identical to the electron in charge, spin and interactions, but are more massive. Leptons differ from the other basic constituent of matter, the quarks, by their lack of strong interaction. All members of the lepton group are fermions, because they all have half-odd integer spin; the electron has spin 1⁄2.[65]
Fundamental properties
The invariant mass of an electron is approximately 9.109×10−31 kilogram,[7] or 5.489×10−4 atomic mass unit. On the basis of Einstein's principle of mass–energy equivalence, this mass corresponds to a rest energy of 0.511 MeV. The ratio between the mass of a proton and that of an electron is about 1836.[9][66] Astronomical measurements show that Proton-to-electron mass ratio has held the same value for at least half the age of the universe, as is predicted by the Standard Model.[67]
Electrons have an electric charge of −1.602×10−19 coulomb,[7] which is used as a standard unit of charge for subatomic particles. Within the limits of experimental accuracy, the electron charge is identical to the charge of a proton, but with the opposite sign.[68] As the symbol e is used for the elementary charge, the electron is commonly symbolized by e⁻, where the minus sign indicates the negative charge. The positron is symbolized by e+ because it has the same properties as the electron but with a positive rather than negative charge.[7][65]
The electron has an intrinsic angular momentum or spin of 1⁄2.[7] This property is usually stated by referring to the electron as a spin-1⁄2 particle.[65] For such particles the spin magnitude is √3⁄2 ħ.[note 3] while the result of the measurement of a projection of the spin on any axis can only be ±ħ⁄2. In addition to spin, the electron has an intrinsic magnetic moment along its spin axis.[7] It is approximately equal to one Bohr magneton,[69][note 4] which is a physical constant equal to 9.274 009 15(23) × 10−24 joules per tesla.[7] The orientation of the spin with respect to the momentum of the electron defines the property of elementary particles known as helicity.[70]
The electron has no known substructure.[2][71] Hence, it is defined or assumed to be a point particle with a point charge and no spatial extent.[10] Observation of a single electron in a Penning trap shows the upper limit of the particle's radius is 10−22 meters.[72] There is a physical constant called the "classical electron radius", with the much larger value of 2.8179×10−15 m. However, the terminology comes from a simplistic calculation that ignores the effects of quantum mechanics; in reality, the so-called classical electron radius has little to do with the true fundamental structure of the electron.[73][note 5]
There are elementary particles that spontaneously decay into less massive particles. An example is the muon, which decays into an electron, a neutrino and an antineutrino, with a mean lifetime of 2.2×10−6 seconds. However, the electron is thought to be stable on theoretical grounds: the electron is the least massive particle with non-zero electric charge, so its decay would violate charge conservation.[74] The experimental lower bound for the electron's mean lifetime is 4.6×1026 years, at a 90% confidence level.[75]
Quantum properties
As with all particles, electrons can act as waves. This is called the wave–particle duality and can be demonstrated using the double-slit experiment. The wave-like nature of the electron allows it to pass through two parallel slits simultaneously, rather than just one slit as would be the case for a classical particle. In quantum mechanics, the wave-like property of one particle can be described mathematically as a complex-valued function, the wave function, commonly denoted by the Greek letter psi (ψ). When the absolute value of this function is squared, it gives the probability that a particle will be observed near a location—a probability density.[76]
Electrons are identical particles because they cannot be distinguished from each other by their intrinsic physical properties. In quantum mechanics, this means that a pair of interacting electrons must be able to swap positions without an observable change to the state of the system. The wave function of fermions, including electrons, is antisymmetric, meaning that it changes sign when two electrons are swapped; that is, ψ(r1, r2) = −ψ(r2, r1), where the variables r1 and r2 correspond to the first and second electrons, respectively. Since the absolute value is not changed by a sign swap, this corresponds to equal probabilities. Bosons, such as the photon, have symmetric wave functions instead.[76]
In the case of antisymmetry, solutions of the wave equation for interacting electrons result in a zero probability that each pair will occupy the same location or state. This is responsible for the Pauli exclusion principle, which precludes any two electrons from occupying the same quantum state. This principle explains many of the properties of electrons. For example, it causes groups of bound electrons to occupy different orbitals in an atom, rather than all overlapping each other in the same orbit.[76]
Virtual particles
Physicists believe that empty space may be continually creating pairs of virtual particles, such as a positron and electron, which rapidly annihilate each other shortly thereafter.[77] The combination of the energy variation needed to create these particles, and the time during which they exist, fall under the threshold of detectability expressed by the Heisenberg uncertainty relation, ΔE·Δt ≥ ħ. In effect, the energy needed to create these virtual particles, ΔE, can be "borrowed" from the vacuum for a period of time, Δt, so that their product is no more than the reduced Planck constant, ħ ≈ 6.6×10−16 eV·s. Thus, for a virtual electron, Δt is at most 1.3×10−21 s.[78]

While an electron–positron virtual pair is in existence, the coulomb force from the ambient electric field surrounding an electron causes a created positron to be attracted to the original electron, while a created electron experiences a repulsion. This causes what is called vacuum polarization. In effect, the vacuum behaves like a medium having a dielectric permittivity more than unity. Thus the effective charge of an electron is actually smaller than its true value, and the charge decreases with increasing distance from the electron.[79][80] This polarization was confirmed experimentally in 1997 using the Japanese TRISTAN particle accelerator.[81] Virtual particles cause a comparable shielding effect for the mass of the electron.[82]
The interaction with virtual particles also explains the small (about 0.1%) deviation of the intrinsic magnetic moment of the electron from the Bohr magneton (the anomalous magnetic moment).[69][83] The extraordinarily precise agreement of this predicted difference with the experimentally determined value is viewed as one of the great achievements of quantum electrodynamics.[84]
In classical physics, the angular momentum and magnetic moment of an object depend upon its physical dimensions. Hence, the concept of a dimensionless electron possessing these properties might seem inconsistent. The apparent paradox can be explained by the formation of virtual photons in the electric field generated by the electron. These photons cause the electron to shift about in a jittery fashion (known as zitterbewegung),[85] which results in a net circular motion with precession. This motion produces both the spin and the magnetic moment of the electron.[10][86] In atoms, this creation of virtual photons explains the Lamb shift observed in spectral lines.[79]
Interaction
An electron generates an electric field that exerts an attractive force on a particle with a positive charge, such as the proton, and a repulsive force on a particle with a negative charge. The strength of this force is determined by Coulomb's inverse square law.[87] When an electron is in motion, it generates a magnetic field.[88] The Ampère-Maxwell law relates the magnetic field to the mass motion of electrons (the current) with respect to an observer. It is this property of induction which supplies the magnetic field that drives an electric motor.[89] The electromagnetic field of an arbitrary moving charged particle is expressed by the Liénard–Wiechert potentials, which are valid even when the particle's speed is close to that of light (relativistic).

When an electron is moving through a magnetic field, it is subject to the Lorentz force that exerts an influence in a direction perpendicular to the plane defined by the magnetic field and the electron velocity. This centripetal force causes the electron to follow a helical trajectory through the field at a radius called the gyroradius. The acceleration from this curving motion induces the electron to radiate energy in the form of synchrotron radiation.[90][91][note 6] The energy emission in turn causes a recoil of the electron, known as the Abraham-Lorentz-Dirac force, which creates a friction that slows the electron. This force is caused by a back-reaction of the electron's own field upon itself.[92]
In quantum electrodynamics the electromagnetic interaction between particles is mediated by photons. An isolated electron that is not undergoing acceleration is unable to emit or absorb a real photon; doing so would violate conservation of energy and momentum. Instead, virtual photons can transfer momentum between two charged particles. It is this exchange of virtual photons that, for example, generates the Coulomb force.[93] Energy emission can occur when a moving electron is deflected by a charged particle, such as a proton. The acceleration of the electron results in the emission of Bremsstrahlung radiation.[94]

An elastic collision between a photon (light) and a solitary (free) electron is called Compton scattering. This collision results in a transfer of momentum and energy between the particles, which modifies the wavelength of the photon by an amount called the Compton shift.[note 7] The maximum magnitude of this wavelength shift is h/mec, which is known as the Compton wavelength.[95] For an electron, it has a value of 2.43 × 10−12 m.[7] When the wavelength of the light is long (for instance, the wavelength of the visible light is 0.4–0.7 μm) the wavelength shift becomes negligible. Such interaction between the light and free electrons is called Thomson scattering or Linear Thomson scattering.[96]
The relative strength of the electromagnetic interaction between two charged particles, such as an electron and a proton, is given by the fine-structure constant. This value is a dimensionless quantity formed by the ratio of two energies: the electrostatic energy of attraction (or repulsion) at a separation of one Compton wavelength, and the rest energy of the charge. It is given by α ≈ 7.297353×10−3, which is approximately equal to 1⁄137.[7]
When electrons and positrons collide, they annihilate each other, giving rise to two or more gamma ray photons. If the electron and positron have negligible momentum, a positronium atom can form before annihilation results in two or three gamma ray photons totalling 1.022 MeV.[97][98] On the other hand, high-energy photons may transform into an electron and a positron by a process called pair production, but only in the presence of a nearby charged particle, such as a nucleus.[99][100]
In the theory of electroweak interaction, the left-handed component of electron's wavefunction forms a weak isospin doublet with the electron neutrino. This means that during weak interactions, electron neutrinos behave like electrons. Either member of this doublet can undergo a charged current interaction by emitting or absorbing a W and be converted into the other member. Charge is conserved during this reaction because the W boson also carries a charge, canceling out any net change during the transmutation. Charged current interactions are responsible for the phenomenon of beta decay in a radioactive atom. Both the electron and electron neutrino can undergo a neutral current interaction via a Z⁰ exchange, and this is responsible for neutrino-electron elastic scattering.[101]
Atoms and molecules

An electron can be bound to the nucleus of an atom by the attractive Coulomb force. A system of several electrons bound to a nucleus is called an atom. If the number of electrons is different from the nucleus' electrical charge, such an atom is called an ion. The wave-like behavior of a bound electron is described by a function called an atomic orbital. Each orbital has its own set of quantum numbers such as energy, angular momentum and projection of angular momentum, and only a discrete set of these orbitals exist around the nucleus. According to the Pauli exclusion principal each orbital can be occupied by up to two electrons, which must differ in their spin quantum number.
Electrons can transfer between different orbitals by the emission or absorption of photons with an energy that matches the difference in potential.[102] Other methods of orbital transfer include collisions with particles, such as electrons, and the Auger effect.[103] In order to escape the atom, the energy of the electron must be increased above its binding energy to the atom. This occurs, for example, with the photoelectric effect, where an incident photon exceeding the atom's ionization energy is absorbed by the electron.[104]
The orbital angular momentum of electrons is quantized. Because the electron is charged, it produces an orbital magnetic moment that is proportional to the angular momentum. The net magnetic moment of an atom is equal to the vector sum of orbital and spin magnetic moments of all electrons and the nucleus. The nuclear magnetic moment is, however, negligible in comparison to the effect from the electrons. The magnetic moments of the electrons that occupy the same orbital (so called, paired electrons) cancel each other out.[105]
The chemical bond between atoms occurs as a result of electromagnetic interactions, as described by the laws of quantum mechanics.[106] The strongest bonds are formed by the sharing or transfer of electrons between atoms, allowing the formation of molecules.[13] Within a molecule, electrons move under the influence of several nuclei, and occupy molecular orbitals; much as they can occupy atomic orbitals in isolated atoms.[107] A fundamental factor in these molecular structures is the existence of electron pairs. These are electrons with opposed spins, allowing them to occupy the same molecular orbital without violating the Pauli exclusion principle (much like in atoms). Different molecular orbitals have different spatial distribution of the electron density. For instance, in bonded pairs (i.e. in the pairs that actually bind atoms together) electrons can be found with the maximal probability in a relatively small volume between the nuclei. On the contrary, in non-bonded pairs electrons are distributed in a large volume around nuclei.[108]
Conductivity

If a body has more or fewer electrons than are required to balance the positive charge of the nuclei, then that object has a net electric charge. When there is an excess of electrons, the object is said to be negatively charged. When there are fewer electrons than the number of protons in nuclei, the object is said to be positively charged. When the number of electrons and the number of protons are equal, their charges cancel each other and the object is said to be electrically neutral. A macroscopic body can develop an electric charge through rubbing, by the triboelectric effect.[112]
Independent electrons moving in vacuum are termed free electrons. Electrons in metals also behave as if they were free. In reality the particles that are commonly termed electrons in metals and other solids are quasi-electrons—quasi-particles, which have the same electrical charge, spin and magnetic moment as real electrons but may have a different mass.[113] When free electrons—both in vacuum and metals—move, they produce a net flow of charge called an electric current, which generates a magnetic field. Likewise a current can be created by a changing magnetic field. These interactions are described mathematically by Maxwell's equations.[114]
At a given temperature, each material has an electrical conductivity that determines the value of electric current when an electric potential is applied. Examples of good conductors include metals such as copper and gold, whereas glass and Teflon are poor conductors. In any dielectric material, the electrons remain bound to their respective atoms and the material behaves as an insulator. Most semiconductors have a variable level of conductivity that lies between the extremes of conduction and insulation.[115] On the other hand, metals have an electronic band structure containing partially filled electronic bands. The presence of such bands allows electrons in metals to behave as if they were free or delocalized electrons. These electrons are not associated with specific atoms, so when an electric field is applied, they are free to move like a gas (called Fermi gas)[116] through the material much like free electrons.
Because of collisions between electrons and atoms, the drift velocity of electrons in a conductor is on the order of millimeters per second. However, the speed at which a change of current at one point in the material causes changes in currents in other parts of the material, the velocity of propagation, is typically about 75% of light speed.[117] This occurs because electrical signals propagate as a wave, with the velocity dependent on the dielectric constant of the material.[118]
Metals make relatively good conductors of heat, primarily because the delocalized electrons are free to transport thermal energy between atoms. However, unlike electrical conductivity, the thermal conductivity of a metal is nearly independent of temperature. This is expressed mathematically by the Wiedemann-Franz law,[116] which states that the ratio of thermal conductivity to the electrical conductivity is proportional to the temperature. The thermal disorder in the metallic lattice increases the electrical resistivity of the material, producing a temperature dependence for electrical current.[119]
When cooled below a point called the critical temperature, materials can undergo a phase transition in which they lose all resistivity to electrical current, in a process known as superconductivity. In BCS theory, this behavior is modeled by pairs of electrons entering a quantum state known as a Bose–Einstein condensate. These Cooper pairs have their motion coupled to nearby matter via lattice vibrations called phonons, thereby avoiding the collisions with atoms that normally create electrical resistance.[120] (Cooper pairs have a radius of roughly 100 nm, so they can overlap each other.)[121] However, the mechanism by which higher temperature superconductors operate remains uncertain.
Electrons inside conducting solids, which are quasi-particles themselves, when tightly confined at temperatures close to absolute zero, behave as though they had split into two other quasiparticles: spinons and holons.[122][123] The former carries spin and magnetic moment, while the latter electrical charge.
Motion and energy
According to Einstein's theory of special relativity, as an electron's speed approaches the speed of light, from an observer's point of view its relativistic mass increases, thereby making it more and more difficult to accelerate it from within the observer's frame of reference. The speed of an electron can approach, but never reach, the speed of light in a vacuum, c. However, when relativistic electrons—that is, electrons moving at a speed close to c—are injected into a dielectric medium such as water, where the local speed of light is significantly less than c, the electrons temporarily travel faster than light in the medium. As they interact with the medium, they generate a faint light called Cherenkov radiation.[124]
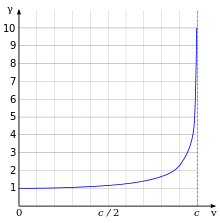
The effects of special relativity are based on a quantity known as the Lorentz factor, defined as 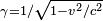 where v is the speed of the particle. The kinetic energy Ke of an electron moving with velocity v is:
where v is the speed of the particle. The kinetic energy Ke of an electron moving with velocity v is:
where me is the mass of electron. For example, the Stanford linear accelerator can accelerate an electron to roughly 51 GeV.[125] This gives a value of nearly 100,000 for γ, since the mass of an electron is 0.51 MeV/c2. The relativistic momentum of this electron is 100,000 times the momentum that classical mechanics would predict for an electron at the same speed.[note 8]
Since an electron behaves as a wave, at a given velocity it has a characteristic de Broglie wavelength. This is given by λe = h/p where h is the Planck constant and p is the momentum.[47] For the 51 GeV electron above, the wavelength is about 2.4×10−17 m, small enough to explore structures well below the size of an atomic nucleus.
No comments:
Post a Comment